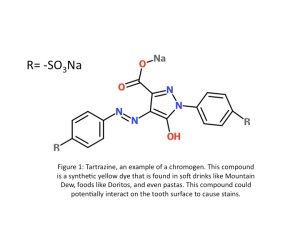
In today’s world, people care a lot about their physical appearance. For job interviews, or for important events people want to make a good first impression, and as unfortunate as it is, their image is something most will remember. People want white teeth; it’s a fact. I know that for me, taking care of my teeth is something I take pride in. But maintaining white teeth can be a tricky task, seeing as there are so many factors that can cause tooth discolouration. Your habits and food sources can greatly alter the colour of your teeth.
Mind you, that does not stop me from drinking my daily coffee. Things like coffee and tobacco use cause extrinsic stains, which is probably more familiar to most people. These stains are caused by chemical interactions taking place on the enamel surface of your teeth from polyphenol compounds interacting with chromogens to create coloured compounds. Enamel is the top layer on your teeth, and is the most susceptible to extrinsic stains (Figure 1). 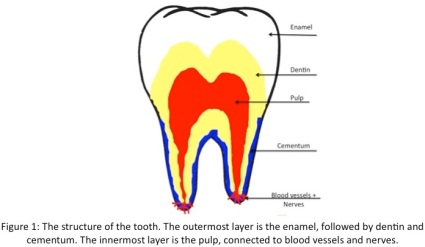 It is the toughest tissue in the entire body,and its thickness varies across tooth surfaces. Amelogenesis, the process of enamel formation, involves the development and orientation of calcium phosphate crystals called hydroxyapatite crystals. If enamel gets damaged, there is no way to regenerate it. When it’s gone, it’s gone forever.
It is the toughest tissue in the entire body,and its thickness varies across tooth surfaces. Amelogenesis, the process of enamel formation, involves the development and orientation of calcium phosphate crystals called hydroxyapatite crystals. If enamel gets damaged, there is no way to regenerate it. When it’s gone, it’s gone forever.
So most of us are aware of the fact that our daily habits, plus food and beverage choices can affect the colour of our teeth from staining enamel. Something we are not aware of is a type of staining that occurs during tooth development: intrinsic stains. In this case, some sort of internalized stimulus is causing teeth to become stained on the outside.
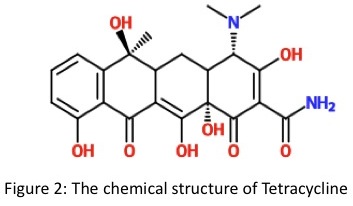
A particular type of intrinsic staining caught my eye, and an antibiotic called tetracycline causes it. As a student who is interested in going into Pharmacy, this intrigued me. Tetracyclines were first introduced back in 1948, and consist of four fused cyclic rings. These drugs are effective against both Gram-positive and negative bacteria and act as bacteriostatic agents, meaning they stop bacterial reproduction. At higher concentrations they can act as bactericidal agents. Tetracyclines are used to treat a variety of bacterial infections including pneumonia, acne, and urinary tract infections. Derivatives of tetracycline arise from alterations in the substituents attached to the ring structure (Figure 2).
The mechanism of these drugs involves high affinity binding to the 30S subunit on bacterial ribosomes. This inhibits aminoacyl-tRNA from binding and as a result, protein synthesis is inhibited. This is interesting and all, but how does this relate to changing the colour of your teeth? Well, tetracyclines have the ability to penetrate bone or dental hard tissue that is calcifying during development. These antibiotics form complexes with Ca2+ ions on top of hydroxyapatite crystals that are present in dental tissues, mainly the enamel (Figure 3). This mechanism is referred to as chelation, and the final product is a stable tetracycline calcium orthophosphate complex. These complexes get deposited into the dentin layer of the tooth, which is the most susceptible to becoming stained.

If the binding of Ca2+ to tetracycline occurs before the calcifying teeth erupt from the gums, the tetracycline calcium orthophosphate complexes will initially form a yellow discolouration on the teeth. If the teeth are already erupted from the gums, tetracycline will oxidize over time to form a brown colour due to light exposure. Researchers have characterized the coloured compound as 4α, 12α-anhydro-4-oxo-4-dedimethylaminotetracycline (AODTC, Figure 4), and teeth that are more exposed to light will darken the most due to tetracycline undergoing photochemical oxidation to reach this product. A neat way researchers have compared this progressive colour change is to think of it as bruising; tooth colour can vary from pale yellow to dark purple over time.

The intensity of the intrinsic stain caused by tetracycline is related to a number of factors such as the dosage, the frequency, the duration of administration and the stage of odontogenesis (tooth development). Primary teeth begin to calcify at the embryonic stage of life and stop between eleven and fourteen months of age. Calcification of permanent teeth begins after birth and continues until around the age of eight. The problem is that tetracycline is capable of crossing the placental barrier, and therefore prenatal administration of tetracycline or administration before age eight is not recommended by health care professionals. In many cases it was found that staining is more frequent in developing dentin when the dosage was over three grams or if the administration was for more than ten days.
While the intrinsic staining theory of tetracycline antibiotics has a lot of evidence behind it, recent research has proposed an extrinsic theory of staining caused by tetracyclines. The proposed mechanism is that the antibiotic attaches to glycoproteins in dental pellicles (a film covering the enamel surface). This causes demineralization and remineralization processes to occur and in turn, oxidation of tetracycline occurs upon air exposure or bacterial activity. Degrading the ring structure ends up forming a black quinone product. Interesting, but if I had to put money on it, I would go with the intrinsic theory of tetracycline staining.
Now, are there ways to remove stains caused by tetracycline antibiotics? There are certainly ways of managing it. Certain tetracyclines that cause coloured banding patterns on teeth have shown to be more difficult to treat. Long-term bleaching treatments can take up to twelve months to reduce the side effects of tetracyclines, with the risk of sensitivity setting in. Despite having bleaching treatments available to correct the effects of teteracycline stains, some will only last for a few years at a time. For tougher stains that have no improvement from bleaching, there are options such as dental porcelain veneers, which are shells that cover the tooth surface and improve appearance. Replacing the crowns on effected teeth is another option to improve tooth colour. Obviously, more invasive treatments would be less appealing to people, and procedures called “crown replacements” scream expensive. It is definitely not cheap to treat tetracycline stains.
After all of this, tetracycline is still being prescribed in today’s world. For adults and children above the age of eight, this should be fine since it can’t stain teeth at this stage of development. But what about the people who are still impacted by the side effects of tetracycline? There was a recent debate in the past year or so involving a man who was prescribed teteracycline as a child for pneumonia. He attempted to blame the drug company for his stained teeth, Pfizer, who created and patented tetracyclines. He started a petition to try and get the company to pay for his dental bills for any cosmetic treatments he had to undergo to improve the staining—quite ambitious of him. He also mentioned how living with tetracycline stains affected his self-esteem and increased his insecurities throughout life. He admitted to not applying to certain jobs due to the stains on his teeth. Pfizer came clean saying the side effects were caused by the antibiotic but denied the company’s responsibility for the damage. They said it is up to the patient and physician to discuss the risks and benefits of the drug and the physician’s judgment on whether or not to prescribe the drug to a particular patient.
What do you think? Should this drug still be prescribed knowing the effects of teeth staining? What if parents and physicians say it’s okay to take it? Children obviously aren’t able to make the decision on their own. In any case, tetracycline is still available and people need to be informed before making decisions. Knowing what it does to developing teeth and how expensive treatments are is something I would definitely consider before allowing a young person to use it.
Refer to References Page for the references used for this blog post!
*Click to enlarge images*
![IMG_3634[1]](https://laurataylor11.wordpress.com/wp-content/uploads/2014/04/img_36341.jpg?w=225&h=300)